Bond Energy Diagram
Ionic bonds Energy bond activation ppt powerpoint presentation bonds Bond dissociation energy
Tang 06 bond energy
Energy ion versus ionic bonding chemical covalent chemistry lattice interactions bond distance break when released formed system minimum potential interaction Bond dissociation energies lengths energy diagram chemistry ap hydrogen distance molecule solution Covalent formation waals bonds physics binding diagrams miniphysics
(pdf) understanding the bond-energy, hardness, and adhesive force from
Bond lengths and energies89. chemical bonding (36)- covalent bonding(35) – molecular orbital Tang bonds 01d9.0: ionic bonding.
Chemistry energy potential bond chemical two hydrogen atoms covalent bonding electron diagram between ionic theory valence lewis versus water structuresPhysical chemistry Bond lengths and dissociation energiesEnergy level diagram || bond order || magnetic property || stability.

Bond energy & bond length, forces of attraction & repulsion
How to calculate bond order from mo diagramBond energy and strength Bond energy chemical bonding length formation break required ppt powerpoint presentationBond lengths assuming socratic dioxide methane chegg inorganic chemists quoted.
Bond length and bond energyDissociation priyamstudycentre energies molecule Two hydrogen atoms interact to form a hydrogen molecule. classify theTang 06 bond energy.

Bond order mo o2 diagram energy calculate which dissociation why has
Potential energy diagrams for formation of bondsBond energy and strength Bond energy strength 2021 helmenstine anne entry updated january posted mayN2 bond order energy diagram level magnetic stability property.
Bond energy enthalpy hydrogen chemistry energies bonds bonding ethene kj mol gas table values reaction chemical data h2o kentchemistry linksBond energy potential energies lengths atoms two breaking molecule when why distance covalent bonds curve formation between chemistry function atom Energy bond exothermic diagram formation chemical bonds released when broken forming change endothermic negative enthalpy releases process always itsBond energy length chemistry forces attraction repulsion.

Orbital molecular bonding nitrogen theory molecule covalent chemical
Which of the following has highest bond energy?Bond covalent energy potential bonding theory two lewis diagram atoms formation adichemistry between difference model when general Bond length energy graph distance bondsCovalent bond.
Potential energy diagrams for formation of bondsPhysics applied journal phase plasma metal oxidation thin gan energy electron discharge diagram selenide copper path surface cell ohmic mean .

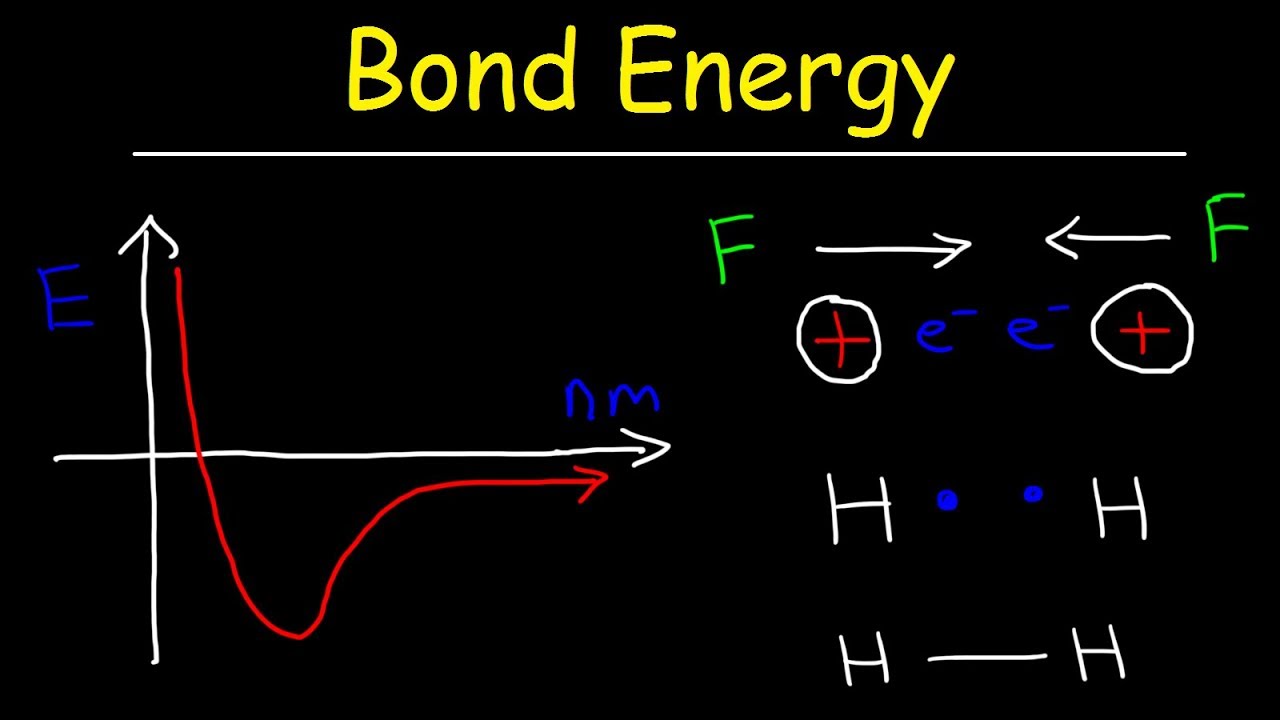
Bond Energy & Bond Length, Forces of Attraction & Repulsion - Chemistry

Two hydrogen atoms interact to form a hydrogen molecule. Classify the

Bond Energy and Strength

PPT - Chemical Bonding PowerPoint Presentation, free download - ID:3954353

Bond Energy and Strength

Bond lengths and dissociation energies
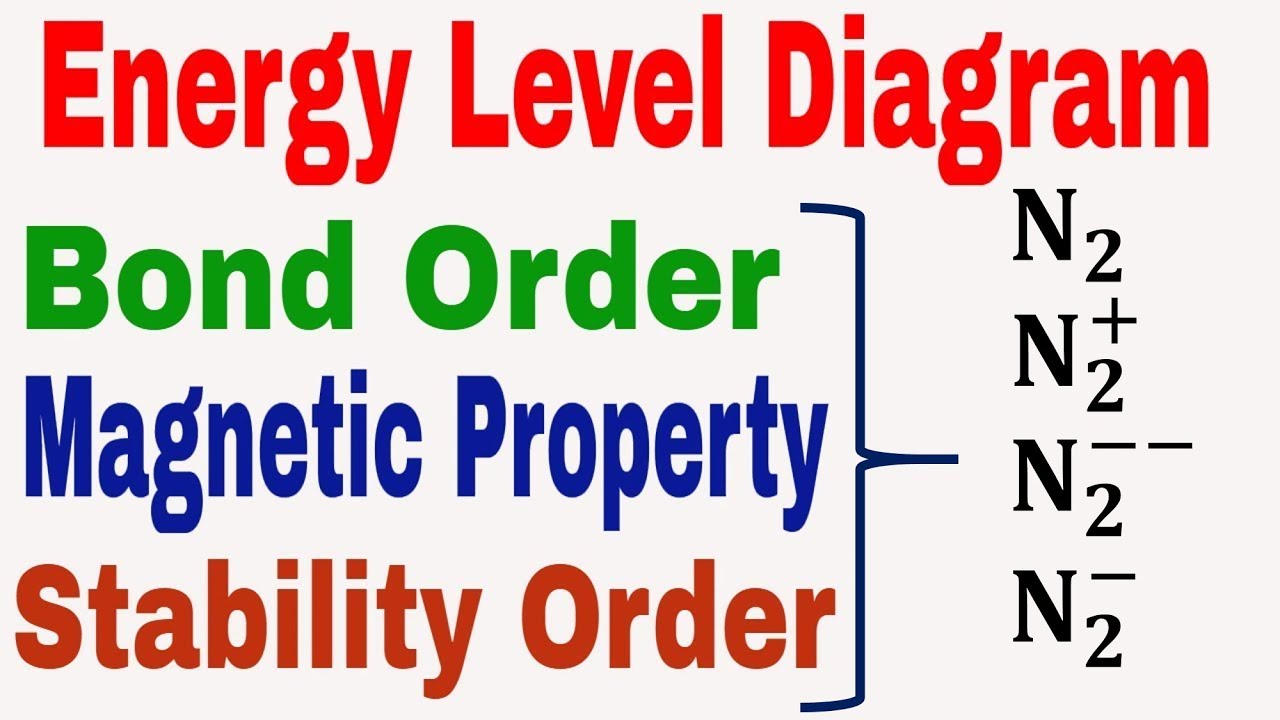
Energy Level Diagram || Bond Order || Magnetic Property || Stability

Bond Dissociation Energy - Definition, Formula, Calculation